Creutzfeldt-Jakob disease in man co-infected with SARS-CoV-2-virus, and Human Herpesvirus type 7
UDK 616.98:578.89+578.834:578.825
DOI : https://doi.org/10.61948/prevmed-2023-4-41
P. A. Dyachenko¹, А. G. Dyachenko²
1. SI “L. V. Hromashevskyi institute of epidemiology and infectious diseases of NAMS of Ukraine”, Kyiv, Ukraine
2. Sumy State University, Sumy, Ukraine
Сreutzfeldt-Jakob disease is a rapidly progressive dementia associated with various histologic changes in brain tissue, including spongiform degeneration, reactive gliosis involving microglia and astrocytes, and neuronal death, sometimes accompanied by amyloid plaque deposition. These changes are believed to be caused by genetic or post-translational modification of a normal cellular protein (PrPC) into an infectious isoform (PrPSc) with highly unusual properties, including the ability to transmit the pathogen to healthy people.
Sporadic Creutzfeldt-Jakob disease is the most common form (more than 80% of all cases of transmissible spongiform encephalopathy). The variant of CJD is less common. The diagnosis of human prion diseases in life is difficult due to the overlapping clinical syndromes. Nevertheless, due to modern advances in lifetime diagnosis, some clinical criteria for CJD have been developed. The most common symptoms are rapidly progressive dementia, myoclonus, akinetic mutism, and signs of cerebellar dysfunction. A wide range of systemic inflammatory mediators characteristic of COVID-19 and HHV-7 infection may have accelerated the pathogenesis of prion disease and neurodegeneration, contributing to the loss of brain cells in both the cortex and white matter.
In addition, the inflammatory process stimulates the production of proinflammatory cytokines and other factors, which in turn activate microglia, which affects the spread of prions.
We describe a man, who suffered from Creutzfeldt-Jakob disease and also was co-infected with SARS-CoV-2-virus both with Human herpesvirus-7. As a result, cognitive and other neuro-pathological disorders progressed to advanced stages very quickly, resulting in death in less than one year after first symptom onset. This case demonstrates the synergy of several pathogens with different mechanisms of nervous system damage.
Key words: Creutzfeldt-Jakob disease, COVID-19, Human Herpesviruses 7.
П. А. Дьяченко¹, А. Г. Дьяченко²
Хвороба Крейцфельдта-Якоба у людини, коінфікованої вірусом SARS-CoV-2 та Герпесвірусом людини типу 7
1. ДУ «Інститут епідеміології та інфекційних хвороб імені Л. В. Громашевського НАМН України, Київ, Україна
2. Сумський державний університет, м. Суми
Хвороба Крейтцфельдта-Якоба — це швидко прогресуюча деменція, пов’язана з різними гістологічними змінами в тканині головного мозку, включаючи губчасту дегенерацію, реактивний гліоз із залученням мікроглії та астроцитів і загибель нейронів, що іноді супроводжується відкладенням амілоїдних бляшок. Вважається, що ці зміни спричинені генетичною або посттрансляційною модифікацією нормального клітинного білка (PrPC) в інфекційну ізоформу (PrPSc) з дуже незвичайними властивостями, включаючи здатність передавати патоген здоровим людям.
Спорадична хвороба Крейтцфельдта-Якоба є найпоширенішою формою (понад 80% усіх випадків трансмісивної губчатої енцефалопатії). Варіант ХКЯ зустрічається рідше. Діагностика пріонних захворювань людини прижиттєво ускладнена через перекриття клінічних синдромів. Однак, завдяки сучасним досягненням у прижиттєвій діагностиці були розроблені деякі клінічні критерії ХБС. Найчастішими симптомами є швидко прогресуюча деменція, міоклонус, акінетичний мутизм та ознаки дисфункції мозочка. Широкий набір системних медіаторів запалення, характерних для COVID-19, та інфекція HHV-7, можливо, прискорили патогенез пріонної хвороби та нейродегенерацію, сприяючи втраті клітин головного мозку, як у корі, так і в білій речовині. Крім того, запальний процес стимулює продукцію прозапальних цитокінів та інших факторів, які зі свого боку активують мікроглію, що впливає на розповсюдження пріонів.
Практикуючому лікарю
Нами описано випадок, коли пацієнт, який страждав на хворобу Крейтцфельда-Якоба, був інфікований одночасно вірусом SARS-CoV-2 і вірусом герпесу людини 7 типу.
Як наслідок, когнітивні та інші невропатологічні розлади дуже швидко прогресували, що призвело до смерті менше ніж через рік після появи перших симптомів. Цей випадок демонструє синергію декількох патогенів із різним механізмом ураження нервової системи.
Ключові слова: хвороба Крейтцфельда-Якоба, COVID-19, вірус герпесу людини 7 типу.
Prion diseases are rare invariably fatal neurodegenerative disorders that most commonly occur sporadically, but can be inherited or acquired,
and for which there is no effective treatment. The causative agent of the disease is believed to have unique biological properties, including ability to overcome the interspecies` barrier.
Case presentation and dicussion
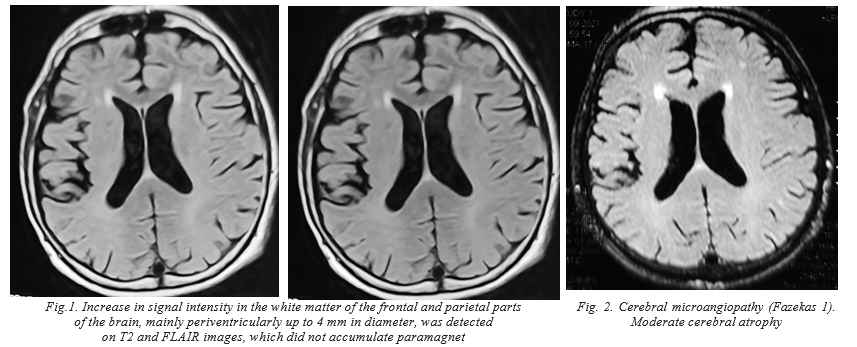
A 60-year-old man was admitted to neurological department of the local hospital with about one-year history of cognitive deterioration and functional decline. He fell ill in July 2021 when aggressiveness, inappropriate behavior, deterioration of long-term memory appeared. In August, confusion of speech appeared, and in a month, he stopped talking. He worked as a cook in a cafeteria, preparing mainly meat dishes. He received a diagnosis of rapidly progressive severe dementia syndrome, and was treated from 30 September to 9 November with no results. After that he was sent to the Center of Neuroinfections for a consultation. During the examination, the patient was disoriented, inattentive, and disinhibited, and reached no verbal contact. Neurologic examination revealed weak convergence, asymmetric nasolabial folds, tongue deviation to the right, horizontal gaze-evoked nystagmus, Marinescu-Radovici reflex and Bekhterev`s reflex (oral automatism) were positive. Muscle tone was increased according to the extrapyramidal type, tendon reflexes D≥S, Babinski reflex was positive on both sides, the patient did not perform coordinating tests. Uncontrolled contractions of the muscles of the shoulder girdle were also noticed; the function of the pelvic organs was not controlled, there were no meningeal signs. A series of the brain MR-images were performed in axial, sagittal and coronary projections in T1, T2 33, FLAIR and DWI modes, as well as T1 33 after intravenous contrast. An increase in signal intensity in the white matter of the frontal and parietal parts of the brain, mainly periventricularly up to 4 mm in diameter, which did not accumulate paramagnet, was detected on T2 and FLAIR images. Diffusion-weighted images showed no pathological increase in the signal from these lesions (fig. 1, 2).
Electroneuromyography revealed an increase in muscle tone by the type of gear wheel. 10,000 copies per 1 ml of Human Herpesvirus 7 DNA were detected by PCR in blood sample. PCR test on SARS-CoV-2 viral RNA was also positive. Cerebrospinal fluid was acellular, with normal opening pressure, protein, glucose, bacterial culture, normal level phospho-tau protein (42.8 pg/ml), beta-amyloid 42/40 ratio, and negative PCR to herpesviruses; positive for IgG CMV and HSV1/2, 14-3-3 protein, tau (9970 pg/ml) protein. CSF and serum autoimmune encephalopathy panels were negative. Given the patient’s profession (butcher), rapid clinical deterioration, imaging findings, and cerebrospinal fluid markers, he was diagnosed with Creutzfeldt–Jakob disease, SARS-CoV-2 infection, activation of HHV-7 infection; dementia, bilateral pyramidal insufficiency, akinetic-rigid syndrome, myoclonus, manifestations of akinetic mutism. The patient died at home in month after the consultation, due to the bleeding from an aneurysm of the superior mesenteric artery.
Creutzfeldt–Jakob disease is a rapidly progressive dementia associated with various histological changes in the brain tissue, including spongiform degeneration, reactive gliosis, involving microglia and astrocytes, and neuronal death, sometimes accompanied with amyloid plaque deposits. These changes are believed to be caused by a genetic or post-translational modification of a normal cellular protein (PrPC) into an infectious isoform (PrPSc) with extremely unusual properties, including ability to transmit pathogen to healthy persons. After transmission or transconformation, they demonstrate long incubation periods, during which prions accumulate in lymphoid tissues, and central nervous system that ultimately trigger neuronal death. The relationship between appearance of this modified protein and prion diseases in animals and humans has been proven. Moreover, immunohistochemical detection of this protein is currently an essential component in the diagnosis of the prion diseases. CJD incidence is 0.5–1 on 1,000 000 people but some cases can stay undiagnosed. There are sporadic (Creutzfeldt–Jakob disease (sCJD), hereditary, and acquired/infectious, usually known as variant (vCJD), forms of these diseases. Sporadic Creutzfeldt–Jakob disease is the most widely distributed form (more than 80% of all cases of transmissible spongiform encephalopathy). Variant CJD is less frequent. The diagnosis of human prion diseases is difficult during life time because of overlapping clinical syndromes. Nevertheless, due to modern achievements in diagnostic premortem techniques, some clinical criteria for CJD were developed. The most frequent symptoms are rapidly progressive dementia, myoclonus, akinetic mutism and signs of cerebellum dysfunction [1]. Rapidly progressive cognitive decline in less than 2 years period is an absolute requirement for the diagnosis of probable CJD. In the presented case, after initial symptoms registered, cognitive and others neuro-pathological disorders progressed to advanced stages very quickly to death in less than one year after symptom onset. The diagnosis was confirmed at autopsy. It should be noted that MRI patterns did not meet expectations, which indicates the currently dominant diagnostic criteria have only relative validity [2–4]. The simultaneous clinical presentations of COVID-19, HHV-7, and CJD in this patient led us to hypothesize that the wide set of systemic inflammatory mediators that characterize COVID-19, and HHV-7 infection may have accelerated the prion disease pathogenesis and neurodegeneration by facilitating loss of brain cells, both in cortex, and white matter. Besides, the inflammatory process stimulates the production of pro-inflammatory cytokines and other factors which in turn activate microglia affected prion propagation [4–10]. In addition, it was previously shown that HHV-7 is one of the main causative agents of encephalitis [11, 12].
Learning points/points of interest
- This case is of interest as a result of co-infection of three pathogens of different classes, the combination of which determined the clinical picture of the disease and complicated the
- The combination of different pathogens entails difficulties in
- Full and competent collection of the epidemiological data and life history improves the chances of correct
- Acute inflammation and immunosuppression caused by COVID-19 worsens the course and prognosis of the underlying
Conclussion
A comprehensive clinical, radiological and virological analysis of the Creutzfeldt-Jakob disease-associated case complicated with COVID-19, and HHV-7-infection, was presented.
Conflict of interest: The Authors declare no conflict of interest.
References
- Zerr , and Poser S. Clinical diagnosis and differential diagnosis of CJD and vCJD. APMIS. 2002; 110(1): 88–98. doi: 10.1034/j.1600-0463.2002.100111.x.
- Rudge P., Hyare H., Green A. et al. Imaging and CSF analyses effectively distinguish CJD from its J Neurol Neurosurg Psychiatry 2018; 89: 461–466. doi:10.1136/jnnp-2017-316853
- Narula R., Tinaz S. Creutzfeldt–Jakob Disease. N Engl J Med, 2018 Jan 25;378(4): doi: 10.1056/NEJMicm1710121
- Young M. J., O’Hare M., Matiello M., Schmahmann J. D. Creutzfeldt-Jakob disease in a man with COVID-19: SARS-CoV-2-accelerated neurodegeneration? Brain, Behavior, and Immunity 89 (2020) 601– https://doi.org/10.1016/j.bbi.2020.07.007
- Liddelow S. A., Guttenplan K. A., Clarke L. E. [et al.]. Neurotoxic reactive astrocytes are induced by activated Nature 2017; 541: 481–487. http://refhub.elsevier.com/S0889-1591(20)31522-1/h0030
- Wu Xu X., Chen Z., Duan J., Hashimoto K. [et al.]. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. 2020 Jul; 87: 18–22. doi: 10.1016/j. bbi.2020.03.0311591(20): 30357-3.
- Steardo , Steardo L. Jr, Zorec R., Verkhratsky A. [et al.]. Neuroinfection may contribute to pathophysiology and clinical manifestations of COVID-19. Acta Physiol (Oxf). 2020 Jul; 229(3): e13473. doi: 10.1111/apha.13473.
- Zanin L Saraceno G., Panciani P. P., Renisi G., Signorini L. [et al.]. SARS-CoV-2 can induce brain and spine demyelinating Acta Neurochir (Wien). 2020 Jul; 162(7): 1491-1494. doi: 10.1007/ s00701-020-04374-x.
- Kemp S. A., Collier D. A., Datir R. P. [et al.] SARS-CoV-2 evolution during treatment of chronic Nature 2021; 592:277–282. https://doi.org/10.1038/s41586-021-03291-y.
- Merli , Perricone G., Lauterio A., Prosperi M., Travi G., Roselli E. et al. Coronaviruses and immunosuppressed patients: the facts during the third epidemic. Liver Transpl. 2020; 26: 1543–4. doi: 10.1002/ lt.25806
- Dyachenko A. Acute Human Herpesvirus-7-associated Encephalitis in a Young Adult Coinfected with Herpes Simplex Virus-1/2 and Epstein-Barr Virus. International Neuropsychiatric Disease Journal. 3 July 2017; 9 (3): 1–5. DOI: 10.9734/INDJ/2017/34258. ISSN:2321–7235
- Dyachenko P. A. and Dyachenko A. G. The Spectrum of Herpesvirus Infections of the Nervous System in Adult Patients in Ukraine: A Prospective Single Center International Neuropsychiatric Disease Journal (INDJ), 2017; 9(4): 1–10, Article no. INDJ.35179. DOI: 10.9734/INDJ/2017/35179
Information about the authors:
Dyachenko P. A. — PhD of medicine, head of the department of neuroinfection Cеntег of infectious disorders of the nervous system, SI “L. V. Hromashevskyi institute of epidemiology and infection diseases of NAMS of Ukraine”
Е-mail: padyac@gmail.com ORCID 0000-0002-0459-9861
Dyachenko A. G. — doctor of medical sciences, professor of Sumy State University
Відомості про авторів:
Дьяченко П. А. — к. м. н., завідувач відділу нейроінфекції Центру інфекційних уражень нервової системи ДУ «Інститут епідеміології та інфекційних хвороб імені Л. В. Громашевського НАМН України».
Е-mail: padyac@gmail.com ORCID 0000-0002-0459-9861
Дьяченко А. Г. — д. м. н., професор Сумського державного університету.
