Impact of IFNL-3 (IL-28B) polymorphism on the kinetics of HBV DNA and qHBsAg and HBsAg clearance during therapy with peginterferon α-2a in patients with HBeAg-negative chronic hepatitis B, genotype D
УДК 616.36-002.2.+616-06:577.245
DOI : https://doi.org/10.61948/prevmed-2024-1-31
V. Fedorchenko, T. L. Martynovych, Zh. B. Klimenko,V. Liashok, I. V. Solianyk, V. A. Reznyk.
SI “The L. V. Hromashevskyi Institute of Epidemiology and Infectious Disease of NAMS of Ukraine”
Corresponding Author: Sergii V. Fedorchenko, Department of Viral Hepatitis and AIDS, SI ”The L. V. Hromashevskyi Institute of Epidemiology and Infectious Diseases of NAMS of Ukraine” Amosova str. 5a, Kiev, 03038, Ukraine.
Tel: +38098 103 76 21; e-mail: fedorchenkosv@i.ua
Abstract. In recent years baseline predictors of Peg-INF response have been identified, one of the host factors may be include: genetic polymorphism IFNL-3 (IL-28B).
The aim of the study. We investigated the effect of IFNL-3 polymorphism (SNP 12979860, SNP rs8099917) on HBV DNA kinetics, qHBsAg, SVR rate and HBsAg clearance. as one of the positive prognostic factors of SBB induction.
Methods. We investigated the effect of IFNL-3 polymorphism (SNP 12979860, SNP rs8099917) on the kinetics of HBV DNA, qHBsAg, the rate of SVR and HBsAg clearance. 108 patients with HBeAg-negative hepatitis B, genotype D were enrolled into the study.
Results. Of 46 patients with CC SNP 12979860, a decline in the concentration of HBsAg>0.5 log10 at 12 weeks of treatment, was noticed in 23 (50.0%) persons in the group of 61 patients, and in 14 (23.0%) with CT alleles (OR=3.36, 95% CI 1.35–8.4, P<0.005). SVR was achieved in 19 (41, 3%) and 12 (19.7%), respectively (OR=3.35, 95% CI 1.12–7.5, P<0.005). Decline in HBV DNA >2 log10 at week 12 was observed in 42 (91.3%), and in 61 patients with CT — in 54 (88.5%) (OR=1.36,CI 0.32–6.75, P=0,0639). At 24 weeks of therapy, the decline in HBV DNA by 2 log10 in the group of patients with CC was detected in 46 (100%) and in those with CT in 61 patients (100%). SVR was achieved in 20 persons (43.5%) and in 11 (18%), respectively (OR=3.5, 95% CI 1.34–9.31, P<0.005). In 1 patient with TT alleles, a 2 log10 decrease in HBV DNA was recorded at 12 and 24 weeks of therapy. In the group of TT SNP rs8099917 alleles carriers (n=63), a decline in HBsAg concentration >0.5 log10 at week 12 was achieved in 27 (42.8%), in the TG group (n=45) — only in 5 (11.1%) (OR=6.0, 95% CI 1.96–21.74, P<0.001). SVR was documented in 24 (38.1%) and 7 (15.6%), respectively (OR=3.34, 95% CI 1.20–10.19, P<0.01). At 12 weeks of therapy in the group with TT alleles, a 2 log10 decline in HBV DNA was observed in 58 individuals (92.0%), in the group with TG — in 40 (88.8%) (OR=1.45, 95% CI 0.31–6.73 P=0.575). At week 24: in TT — 63 patients (100.0%) and in TG — 45 patients (100.0%). SVR was achieved in 23 (36.5%) and 8 (17.7%), respectively (OR=2.66, 95% CI 0.99–7.7, P<0.05).
Conclusion. The presented study demonstrates that favorable genetic polymorphism IFNL-3 (SNP 12979860 and SNP rs8099917) is one of the most significant baseline positive predictive factors on SVR induction.
Key words HBeAg-negative chronic hepatitis B, peginterferon, IFNL-3 (IL-28B) polymorphism, sustained virological response, HBsAg.
Вплив поліморфізму IFNL-3 (IL-28B) на кінетику ДНК HBV та кліренс qHBsAg та HBsAg під час терапії пегінтерфероном α-2a у хворих на HBeAg-негативний хронічний гепатит В, генотип D.
С. В. Федорченко, Т. Л. Мартинович, Ж. Б. Клименко, О. В. Ляшок, І. В. Соляник, В. А Резник
ДУ «Інститут епідеміології та інфекційних хвороб імені Л. В. Громашевського НАМН України»
Адреса для листування: Федорченко С. В., відділ вірусних гепатитів та СНІД, ДУ «Інститут епідеміології та інфекційних хвороб імені Л. В. Громашевського НАМН України» вул. Амосова,5, Київ, 03038, Україна.
Тел: +380 98 103 76 21; e-mail: fedorchenkosv@i.ua
Анотація. В останні роки були визначені базові предиктори відповіді Peg-INF, одним із факторів хазяїна може бути генетичний поліморфізм IFNL-3 (IL-28B).
Мета. Ми досліджували вплив поліморфізму IFNL-3 (SNP 12979860, SNP rs8099917) на кінетику ДНК HBV, qHBsAg, швидкість SVR і кліренс HBsAg як один із позитивних прогностичних факторів індукції СВВ.
Методи. У дослідження було включено 108 хворих на HBeAg-негативний гепатит В, генотип D.
Результати. Із 46 пацієнтів із СС SNP 12979860 зниження концентрації HBsAg >0,5 log10 через 12 тижнів лікування спостерігалося у 23 (50,0%) осіб у групі 61 пацієнта та у 14 (23,0%) з Алелі CT (OR=3,36, 95% ДІ 1,35–8,4, P<0,005). СВВ було досягнуто у 19 (41, 3%) і 12 (19,7%) відповідно (OR=3,35, 95% ДІ 1,12–7,5, P<0,005). Зниження HBV DNA >2 log10 на 12 тижні спостерігалося у 42 (91,3%), а у 61 пацієнта з ХТ — у 54 (88,5%) (OR=1,36, CI 0,32–6,75, P=0,0639). Через 24 тижні терапії зниження ДНК HBV на 2 log10 у групі хворих на СС виявлено у 46 (100 %), а з СТ — у 61 пацієнта (100%). СВВ було досягнуто у 20 осіб (43,5%) і в 11 (18%) відповідно (OR=3,5, 95% ДІ 1,34–9,31, P<0,005). В одного пацієнта з алелями ТТ було зареєстровано зниження ДНК HBV на 2 log10 на 12 і 24 тижні терапії. У групі носіїв алелів TT SNP rs8099917 (n=63) зниження концентрації HBsAg >0,5 log10 на 12 тижні досягнуто у 27 (42,8%), у групі TG (n=45) — лише у 5 (11,1%) (OR=6,0, 95% ДІ 1,96–21,74, P<0,001). СВВ було задокументовано у 24 (38,1%) і 7 (15,6%) відповідно (OR=3,34, 95% ДІ 1,20–10,19, P<0,01). Через 12 тижнів терапії в групі з алелями ТТ зниження ДНК HBV на 2 log10 спостерігалося у 58 осіб (92,0%), у групі з ТГ — у 40 (88,8%) (OR=1,45, 95% ДІ 0,31–6,73 P=0,575). На 24 тижні: в ТТ — 63 хворих (100,0%) і в ТГ — 45 пацієнтів (100,0%). СВВ було досягнуто у 23 (36,5%) та 8 (17,7%) відповідно (OR=2,66, 95% ДІ 0,99–7,7, P<0,05).
Висновок. Представлене дослідження демонструє, що сприятливий генетичний поліморфізм IFNL-3 (SNP 12979860 і SNP rs8099917) є одним із найбільш значущих базових позитивних прогностичних факторів індукції СВВ.
Ключові слова. HBeAg-негативний хронічний гепатит В, пегінтерферон, поліморфізм IFNL-3 (IL-28B), стійка вірусологічна відповідь, HBsAg.
Introduction.
Identification of the IFNL-3 gene polymorphism (rs12979860, rs12980275, and rs8099917) has been widely used in clinical practice to predict the achievement of a sustained virological response (SVR) in the treatment of chronic hepatitis C virus with interferoncontaining treatment regimens, as well as for the likelihood of spontaneous HCV clearance [1–3]. The study of this genetic polymorphism over the past 8–10 years has not provided unambiguous data on its effect on the natural course of chronic viral hepatitis B (CHB) (rate of HBeAg seroconversion to anti-HBe, HBsAg clearance, and incidence of hepatocellular carcinoma (HCC)) [4, 5]. Peginterferon belongs to the first line of drugs recommended by EASL, AASLD, NICE for the treatment of HBeAg-positive and HBeAg-negative variants of chronic hepatitis B virus. The studies conducted in different regions of the Globe dedicated to the likely influence of IFNL-3 polymorphism on DNA HBV, qHBsAg kinetics and also the clearance of HBsAg vary significantly. Thus, in a study of HBeAg-positive and HBeAg-negative patients in Southeast Asia treated with peginterferon, no effect of IFNL-3 polymorphism (rs12979860, rs12980275 and rs8099917) on the rate of anti-HBe seroconversions, HBsAg clearance was found [6–8]. At the same time, other researchers from this region report a significant effect of genetic polymorphism on the outcomes of peginterferon therapy [9].
Comparison of the results of studies carried out in Southeast Asia and Europe is not entirely correct, since the HBeAg-positive variant of chronic viral hepatitis B, with a vertical transmission route and a dominant HBV C genotype prevails in Asia. Throughout Europe about 80% are diagnosed with HBeAg-negative CHB with a prevalence of the D genotype and horizontal route of transmission [10, 11]. There exist significant ethnic differences in the distribution of IFNL-3 polymorphism in Asian and European patients with CHB [12]. Based on the foregoing, in the future, when discussing the results obtained, we will focus on the research conducted in Europe, with the inclusion of HBeAg-positive and HBeAg-negative patients.
Review of literature data on the possible effect of IFNL-3 polymorphism on the efficacy of peginterferon therapy in European patients with CHB did not give unambiguous results. Thus, in treating HBeAg-negative patients with genotype D with peginterferon, no effect of SNP rs12979860, rs8099917, rs12980275 on the induction of virological and biochemical responses, HBsAg clearance and the rate of anti-HBs seroconversion was observed [13, 14].
At the same time, a large number of studies have been published confirming the correlation between IFNL-3 polymorphism and the rate of SVR induction, HBsAg clearance, both in HBeAg-positive variant of chronic hepatitis B [15] and in HBeAg-negative one [16, 17].
Our aim was to study the effect of SNP IFNL-3 (rs12979860, rs8099917) on the kinetics of HBV DNA, qHBsAg, SVR induction rate, and HBsAg clearance in HBeAgnegative patients with chronic hepatitis B, genotype D during 48 week long therapy with peginterferon α-2a.
Materials and methods. The study was conducted from June 1917 to November 2020 and included 117 HBeAg-negative patients with CHB (108 patients (92.3%) with genotype D, 9 (7.7%) — with genotype A). 81 (75%) males, 27 (25%) females, the average age was 48.6±3.9. Of 108 patients with genotype D, who completed 48 weeks treatment 48, anti-HBe was detected in 79 subjects (73.1%), in the remaining 29 patients HBeAg was not detected. All patients were of Slavonic origin.
Patients inclusion criteria:
- Age of 18 years or older;
- Informed consent signed;
- Level of DNA HBV viremia >2·103 IU/mL, ALT >40 U/mL;
- Genotype D HBV. Patients exclusion criteria:
- Decompensated liver disease, which were marked as Child-Pugh classes B or C;
- Current НСС confirmed by ultrasonography or CT, or history of НСС;
- Co-infection with HDV, HCV or HIV;
- Detected laboratory abnormalities, such as ALT level more then ten times the upper limit of the normal range, platelets count lower than 100 000 mm3 and severe anemia with hemoglobin levels <9.0 g/d;
- Another form of liver disease in addition to viral hepatitis;
- Patients with creatinine clearance <35 ml/min or di-
Assessment of Liver fibrosis. Fibrosis stage was determined by ”FibroScan 502 touch” equipment. Results are measured using kiloPascal’s (kPa) and range from 2 to 75. Stages of fibrosis: F0-F1 — 2–8 kPa, F2 — 8–10kPa, F3 — 10–14 kPa, F4 — 14 or higher. Absence of fibrosis or moderate fibrosis (F0–F2 according to METAVIR) were found in 91 patients (84.3%), severe fibrosis and liver cirrhosis (F3-F4 according to METAVIR) — in 17 patients (15.7%).
Virologic Testing. HBsAg levels were determined by Elecsys HBsAg II Quant reagent kits (Roche Diagnostics, Indianapolis, IN), HBV DNA levels were tested with the qualitative COBAS Amplicor HBV Test (Roche Molecular Systems, Branchburg, NG; lower limit of detection, 50 IU/ mL. HBV genotype was determined using the VERSANT HBV Genotype 2.0 Assay (LiPA; Siemens Medical Solutions Diagnostics, Tarrytown, NY). Antibodies directed against HCV, HDV and HIV infection status were determined by the Aksum system (Abbot. Laboratories, Chicago, IL, USA) by determining anti-HBs (AUSAB), and HBsAg (HBsAgV2). Anti-HIV and anti-HCV were tested by Abbott PRISM (Abbott Laboratories, Chicago, IL. USA). Anti-HBe, HBeAg were determined by Elecsys (Roche Diagnostics, Indianapolis, IN). Anti-HDV were tested by DiaSorin ETI-AB-DELTAK-2. All assays were performed according to the manufacturer`s instructions.
Testing for IFNL-3 (IL-28В). Genomic DNA was isolated from peripheral blood according to the QIAamp DNA Blood Mini Kit from Qiagen (Hilden, Germany). SNPs rs12979860 and rs8099917 in the region of the IL28B gene were analyzed by the StepOnePlus Real-Time PCR System (Applied Biosystems, Foster City, CA) with the help of a custom TaqMan SNP Genotyping Assay developed together with Applied Biosystems. Amplicon sequencing was used to validate the genotyping techniques.
Autoantibody. ANA was performed using ELISA kits (Phadia GmbH, Freiburg, Germany), Anti-LKM1 and AntiSLA were determined by ELISA kits (Eagle Biosciences, Inc. Amherst, NH, USA).
Statistical analysis. Database management and statistical analysis were performed using commercially available software systems (Microsoft Office Excel 2010, Microsoft Corp, Redmond, WA; SPSS 2006 for Windows version 16, SPSS Inc, Chicago, IL; and MedCalc 11.4.2.0, Software bvba, Mariakerke, Belgium). Data were ana- lyzed by using Mann-Whitney U test as well as χ2 test, and the degree of association between independent variables of the favorable SNP IFNL-3(rs12979860, rs8099917) and unfavorable one SVR achieved, was determined by calculation the corresponding odds ratio (OR) and its 95% confidence interval (95% CI) by means of simple logistic regression. Data were analyzed in the univariate analysis according to variables of interest using Fisher’s exact test. Data were analyzed in IBM SPSS Version22; p-values <0.05 were considered statistically significant.
Informed consent. Informed consent was obtained from each patient.
Ethics committee approval. Ethics committee approval was received for this study from the ethics committee of the State Institution “L. V. Hromashevskyi Institute of Epidemiology and Infectious Diseases of the NAMS of Ukraine”.
The diagnosis of chronic hepatitis B virus infection was made based on the presence of HBsAg in the serum at least 12 months before the patient was enrolled in the study. Treatment with peginterferon α-2a — 180 mg once a week, for 48 weeks was carried out in patients with baseline viremia level of >2 • 103 IU/mL, ALT activity >40 U/mL. Coinfection was ruled out on the basis of negative test results for anti-HCV, anti-HIV and anti-HDV [18]. Autoimmune hepatitis was ruled out when results for anti-ANA, anti-LKM-I and anti-SLA were negative.
At the start of therapy all patients were divided into four groups by the IFNL-3 polymorphism rs12979860 CC, and CT, TT, patients were also selected for rs8099917 TT and TG, GG.
The efficacy of peginterferon therapy was assessed by the kinetics of qHBsAg and HBV DNA concentrations every 3 months in each of the groups during 48 weeks of therapy. Laboratory tests were done 48 weeks after the end of treatment to determine the outcomes of therapy. SVR has been documented when HBV DNA was <2×103 IU/mL, ALT activity was normal (N<40 U/mL). There was HBsAg clearance or anti-HBs seroconversion. HBsAg clearance was diagnosed when a negative qualitative test was obtained, there was seroconversion of anti-HBs and the presence of antibodies >10 IU/ml.
The virological response, assessed by the kinetics of qHBsAg and HBV DNA during the first 24 weeks of peginterferon therapy, is decisive for deciding whether the therapy is effective. In the absence of any decline in HBsAg concentration and a decline in HBV DNA level of less than 2 log10 IU/ml at 12 weeks, peginterferon treatment should be discontinued (PARC-rule), since the likelihood of achieving SVR is extremely low [19]. Treatment is terminated early if the HBV DNA level decline is <2 log10 IU/ ml at HBsAg concentration >20,000 IU/ml at 24 weeks of therapy (NICE-rule) [20].
In our study we ignored the stop rules and continued therapy for 48 weeks with the determination of the HBV DNA concentration, qHBsAg at baseline, 12, 24, 36 and 48 weeks of treatment. SVR was documented 48 weeks or more after the end of therapy in 108 patients with HBeAg-negative chronic hepatitis B virus, genotype D. To determine the effect of IFNL-3 polymorphism on DNA HBV kinetics, and qHBsAg we divided our patients into four groups, as mentioned above and also assessed the rate of SVR induction and HBsAg clearance 48 weeks after the end of treatment in each of the groups. Determining HBV DNA concentration and qHBsAg at 12 weeks of therapy is critical to assess the likelihood of achieving SVR. However, the PARC-rule dictate the amount of the required HBsAg concentration decline for using it. On the other hand, some patients in whom peginterferon therapy should be discontinued, based on the stopping PARC-rule, reach SVR if treatment is continued for up to 48 weeks [21]. The study of the qHBsAg kinetics at 12 weeks of peginterferon therapy demonstrated that patients with a decline in HBsAg concentration >0.5 log10 have the highest chances of achieving SVR (NPV — 90%, PPV — 89%) [22]. Based on the above, at 12 weeks, we took into account a decline in HBsAg concentration >0.5 log10, compared to the baseline.
At 24 weeks of treatment we evaluated the efficacy of therapy associated with on the IFNL-3 polymorphism using the PERSEAS-rule (decline in q HBsAg >log10 and DNA HBV >2 log10 [21]. At this stage we evaluated the stopping NICE-rule (decline in DNA HBV <2 log10 IU/ml with an HBsAg concentration >20,000 IU/mL) [20].
Side effects and modification of peginterferon α-2a dosing. Not a single patient had prematurely terminated treatment due to side effects. Anemia in 26 patients (24.1%), headache in 31 patients (28.7%), cytopenia (neutropenia, trombopenia) in 43 patients (39.8%) and diarrhea in 8 patients (7.4%) were registered most often. Due to the development of cytopenia in fifteen patients, the daily dose of peginterferon was reduced to 90–120 mg per week for 2–4 weeks, with subsequent resumption of the drug dose to 180 mg per week. The use of growth factors was allowed (thirty one patients).
Results.
Kinetics of q HBsAg depending on IFNL-3 polymorphism (rs12979860 and rs8099917).
At the first stage of studying the kinetics of qHBsAg, we analyzed how the baseline concentration of HBsAg affects the dynamics of this protein during treatment and achievement of SVR with and without taking into account the IFNL-3 polymorphism. We considered the baseline concentration of HBsAg <2,000 IU/ml low. This baseline low concentration was found in 21 patients (19.4%) of 108. 16 persons (76.2%) from this group achieved SVR and 15 (17.2%) in the group of 87 patients with high HBsAg concentration (OR=15.36, 95% CI 4.37– 60.1, P<0.001).
The next stage of research was dedicated to studying the effect of IFNL-3 polymorphism on the achievement of SVR depending on the baseline concentration of HBsAg. The HBsAg concentration <2000 IU/ml was found in 9 (19,6%) of 46 patients with CC rs12979860 and in 12 (19.7%) of 61 patients with CT rs12979860. SVR was achieved in 9 (100%) patients with CC and in 10 (83.3%) patients with CT alleles. In 37 patients with a high baseline HBsAg concentration, SVR was achieved in 8 patients (21.6%) of 37 patients with CC and in the group of 49 patients with CT in 4 (8.2%). One patient with TT and high HBsAg concentration did not respond to therapy.
In the group of patients with TT SNP rs8099917, the initial low level of qHBsAg was detected in 15 patients (23.8%) out of 63 and in 5 (11.1%) out of 45 with TG. SVR was achieved in 15 patients (100%) with TT and 3 (60%) with the TG allele. In a group of 48 people with TT with a high concentration of HBsAg, SVR was achieved in 9 (18.8%) and in 4 (10%) in those with TG (n=40). The GG allele was not detected in any of the patients.
Finally, we analyzed the kinetics of HBsAg concentration depending on IFNL-3 polymorphism at the start of therapy, 12, 24, 36 and 48 weeks. The first 24 weeks of treatment proved to be decisive in terms of the nature of the response to therapy and the likelihood of achieving SVR.
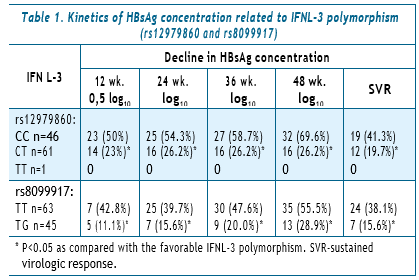
Thus, out of 46 patients with CC SNP rs12979860, a decline in HBsAg concentration >0.5 log10 at 12 weeks of treatment, compared with baseline values, was noticed in 23 persons (50%) and in a group of 61 patients with CT alleles — in 14 (23%) (OR=3.36, 95% CI 1.35–8.4, P<0.005).
At 24 weeks of therapy, a decline in HBsAg concentration >log10 in the CC group was noticed in 25 people (54.3%), and in 16 (26.2%) patients with CT (OR=3.35, 95% CI 1.37–8.2, P<0.01), SVR was achieved in 19 (41.3%) and 12 (19.7%), respectively (OR=3.35, 95% CI 1.12–7.5, P<0.005).
The distribution of patients by SNP rs8099917 also demonstrated a close relationship between genetic polymorphism, qHBsAg kinetics, and the likelihood of achieving SVR. Thus, in the group of TT alleles carriers (n=63), a decline in HBsAg concentration >0.5 log10 at 12 weeks of treatment was achieved in 27 (42.8%), and in only in 5 (11.1%) in the TG group (n=45) (OR=6.0, 95% CI 1.96–21.74, P<0.001).
Kinetics of HBV DNA concentration depending on IFNL-3 polymorphism (rs12979860 and rs8099917). At the start of therapy we considered the HBV DNA concen-tration <6 log10 low. Of the 108 patients enrolled in the study, a low concentration was found in 82 individuals — 75.9%, and high concentration in 26 (24.1%) individuals. Treatment resulted in achievement of SVR in 27 patients with a low concentration (32.9%) and in 4 with a high concentration (15.4%) (OR=7.2, 95% CI 0.80–11.75, P=0.085).
We further proceeded with studying the effect of IFNL-3 genetic polymorphism on SVR achievement depending on the baseline HBV DNA concentration. A low baseline concentration of DNA HBV in the group of patients with rs12979860 CC (n=46) was detected in 32 individuals (69.6%) and with CT (n=61) it was detected in 49 (80.3%) patients. SVR was achieved in 13 individuals (40.6%) with CC and 10 (20.4%) with CT (OR=2.67, CI 0.89–8.1, P<0.05). In a group of 14 CC patients with a high level of HBV DNA, SVR was achieved in 5 (35.7%) in the group of 12 individuals with CT — in 3 (25%) (OR=1.67, CI 0.23–13.85, P=0.555).One patient with TT alleles had low HBV DNA level. In the group of patients with SNP rs8099917, the baseline low level of HBV DNA was detected in 45 (71.4%) of 63 patients with TT and in 29 (64.4%) of 45 patients with TG alleles. SVR was achieved in 20 (44.4%) patients with TT and in 4 (13.8%) with TG (OR=3.13, CI 1.3– 7.55, P<0.005). In the group of 18 people with CC with a high concentration of DNA HBV SVR was achieved in 4 (22.2%), in 16 patients with TG — in 3 (18.7%) (OR=1.23, 0.17–10.1, P=0.803). Finally, we analyzed the kinetics of HBV DNA at baseline, 12 and 24 weeks of therapy, depending on the IFNL-3 polymorphism, and the likelihood of achieving SVR.
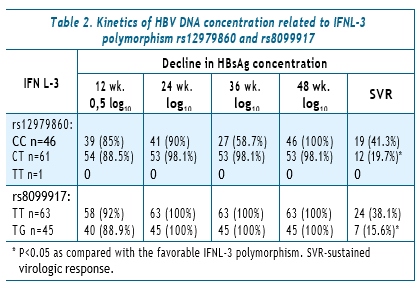
In the group of 46 patients with SNP 12979860 CC, a decline in HBV DNA >2 log10 at week 12 was observed in 42 (91.3%), and in 61 patients with CT — in 54 (88.5%) (OR=1.36, CI 0.32–6.75, P=0.0639). At 24 weeks of therapy, the decline in HBV DNA by 2 log10 in the group of patients with CC was detected in 46 (100%) and in those with CT in 61 patients (100%). SVR was achieved in 20 persons (43.5%) and in 11 (18%), respectively (OR=3.5, 95% CI 1.34–9.31, P<0.005). In 1 patient with TT alleles, a 2 log10 decrease in HBV DNA was recorded at 12 and 24 weeks of therapy.
The effect of SNP rs8099917 polymorphism on the kinetics of HBV DNA concentration at 12 and 24 weeks of peginterferon therapy was studied in 108 people: 63 patients with the TT allele and 45 patients with the TG allele.
At 12 weeks of therapy in the group with TT alleles, a2 log10 decline in HBV DNA was observed in 58 individuals (92.0%), in the group with TG — in 40 (88.8%) (OR=1.45, 95% CI 0.31–6.73 P=0.575). At week 24: in TT — 63 patients (100.0%) and in TG — 45 patients (100.0%). SVR was achieved in 23 (36.5%) and 8 (17.7%), respectively (OR=2.66, 95% CI 0.99–7.7, P<0.05) (Table 2).

IFNL-3 polymorphism (SNP 12979860, SNP rs8099917) and HBsAg clearance. Of 108 patients infected with the D genotype HBV who have been receiving peginterferon therapy for 48 weeks, HBsAg clearance was recorded in 9 (8.3%) persons. Of these, 5 females and 4 males, the average age 34.9±9.4. Portoseptal fibrosis (F3) was diagnosed in only one patient. A baseline low HBsAg level of <2000 IU/ml was recorded in 5 out of 9 patients. At 12 weeks of therapy, a decline in HBsAg concentration >0.5 log10 or a negative qualitative test for HBsAg was documented in 8 out of 9 individuals. At 24 weeks all patients with detectable HBsAg concentration achieved a decline in this protein >log10. All patients showed a decline in HBV DNA of >2 log10 at 12 and 24 weeks. Elimination of HBsAg was noted in all patients during therapy with seroconversion in 2 individuals. In 48 weeks after the end of treatment, seroconversion was documented in 2 more patients. Thus, at 48 weeks, anti-HBs seroconversion was detected in 4 out of 9 persons.
Interesting data were obtained on the effect of IFNL-3 (SNP 12979860, SNP rs8099917) on HBsAg clearance, so the distribution of the favorable CC allele (SNP 12979860) in the general group of patients (n=108) was 46 (42.6%) persons and in patients with HBsAg clearance 7 (77.7%) of 9 patients (OR=4.72, CI 0.84–47.9 P<0.05) were found to be carriers of the CC allele. Favorable polymorphism TT allele (SNP rs8099917), was detected in 8 (88.8%) of 9 patients, while among 108 patients in 63 (58.3%) (OR=5,71, CI 0.71–259.0, P=0.071)
(Table 3).

Discussion.
Peginterferon α-2a therapy for chronic HBeAg-negative hepatitis for 48 weeks often leads to the development of a number of side effects, the most common of them being: flu-like syndrome, headache, myalgia, fatigue, depression, weight loss, hair loss, cytopenia, which significantly reduces the quality of life of patients. In some cases, therapy can lead to life-threatening conditions (the development of autoimmune diseases, mental disorders, decompensation of the process in patients with advanced forms of fibrosis). Peginterferon treatment leads to the induction of SVR in 25–35% of patients with HBsAg clearance in 3–9% [21]. And although the rate of HBsAg clearance 5 years after the end of treatment increases to 12% [22, 23], peginterferon therapy can hardly be called highly effective.
Based on the above, in recent years baseline predictors of Peg-INF response have been identified, according to which it is possible to pre-select patients with HBeAgnegative chronic hepatitis B virus in whom therapy would be most effective. These predictive factors can be roughly divided into the viral factors and the host ones. Positive baseline viral predictive factors include: a low level of HBV DNA, genotypes A or B, and possibly a low baseline concentration of HBsAg [24, 25]. Host factors include: high ALT activity, young age, female sex, and genetic polymorphism [26]. Over the past 10 years, the effect of IFNL-3 on SVR induction, HBsAg clearance during peginterferon therapy in patients with chronic HBeAg-positive and HBeAg-negative hepatitis B has been studied with the greatest frequency.
108 patients with HBeAg-negative variant of hepatitis B, genotype D were enrolled into the study. Anti-HBe was detected in 79 (73.1%) participants. The absence of fibrosis or moderate fibrosis was diagnosed in 91 (84.3%) patients. All patients were divided into four groups for IFNL-3 polymorphism, as previously mentioned.
At the first stage of our research, we studied the effect of the baseline HBsAg concentration on the likelihood of achieving SVR regardless of the IFNL-3 gene polymorphism. Then, the effect of SNP 12979860 and SNP rs8099917 polymorphism and baseline concentration on the achievement of SVR and finally we followed the kinetics of qHBsAg depending on genetic polymorphism at 12, 24, 36 and 48 weeks of peginterferon therapy. The baseline low concentration of HBsAg (<2000 IU/ml) was determined in 21 out of 108 patients (19.4%). As a result of peginterferon treatment resulted in achievement of SVR in 16 (76.2%) and 15 (17.2%) of 87 patients with high HBsAg concentration. The baseline low HBsAg concentration was recorded in 9 (19.6%) of 46 patients with CC SNP 12979860 alleles and in 12 (19.7%) of 61 with CT SNP 12979860. SVR was achieved in 9 (100%) with CC and in 10 (83.3%) individuals with CT alleles.
Of 37 people with CC alleles with a high baseline HBsAg concentration, SVR was achieved in 8 (21.6%) persons and in the group of 49 patients with CT — in 4 (8.2%). One patient with TT allele with high HBsAg concentration did not respond to therapy. The distribution of patients by SNP rs8099917 IFNL-3 polymorphism demonstrated that the initial low level of qHBsAg was detected in 15 (23.8%) individuals out of 63 with the TT allele and in 5 (11.1%) out of 45 patients with the TG allele. SVR was achieved in 15 (100.0%) with TT and 3 (60.0%) with TG alleles.
Based on the data obtained, we concluded that the most favorable predictive factors include a low baseline qHBsAg concentration and a favorable IFNL-3 polymorphism in SNP 12979860 CC and SNP rs8099917 TT, in which all patients achieved SVR. Analyzing the kinetics of qHBsAg at 12 and 24 weeks of treatment and the likelihood of achieving SVR, we can conclude that favorable genetic polymorphism (SNP 12979860 CC and SNP rs8099917 TT) leads to a faster decline in HBsAg concentration during therapy and increases the induction of SVR by more than 2 times compared to the unfavorable IFNL-3 polymorphism.
Of 46 patients with CC SNP 12979860, a decline in the concentration of HBsAg >0.5 log10 at 12 weeks of treatment, compared with the baseline values, was noticed in 23 (50.0%) persons, in the group of 61 patients in 14 (23.0 %) with CT alleles.
At 24 weeks of therapy, a decline in HBsAg >log10 concentration in the CC group was noticed in 25 (54.3%), and 16 (26.2%) in patients with CT, SVR was achieved in 19 (41.3%) and 12 (19.7%), respectively.
The distribution of patients by SNP rs8099917 also demonstrated a close relationship between genetic polymorphism, qHBsAg kinetics, and the likelihood of achieving SVR. In the group of TT alleles carriers (n=63), a decline in HBsAg concentration >0.5 log10 at week 12 was achieved in 27 (42.8%), in the TG group (n=45) — only in 5 (11.1%).
At 24 weeks of therapy, a decline in qHBsAg >log10 in the group with TT was achieved in 25 (39.7%), and in 7 (15.6%) of those with TG. At 48 weeks after the end of treatment, SVR was documented in 24 (38.1%) and 7 (15.6%), respectively.
Of 108 patients with chronic HBeAg-negative hepatitis B, the baseline low concentration of HBV DNA (<6 log10 IU/ml) was detected in 82 (75.9%) persons, and a high one — in 26 (24.1%) persons. SVR was achieved in 27 (32.7%) patients with low concentration and in 4 (15.4%) with high concentration.
The baseline low concentration of HBV DNA in the group of patients with IFNL-3 (SNP 12979860) CC (n=46) was detected in 32 (69.6%) persons, in those with CT (n=61) — in 49 (80.3%) patients. SVR was achieved in 13 (40.6%) persons with CC and 10 persons (20.4%) with CT. Of 14 patients with CC with a high level of DNA HBV SVR was achieved in 5 (35.7%); of 12 with CT — in 3 (25.0%).
The selection of patients by the IFNL-3 polymorphism (SNP rs8099917) did not demonstrate any dependence between the baseline HBV DNA concentration with the groups carrying the TT and TG alleles. Initial low level of viremia was detected in 45 (71.4%) of 63 patients with TT and in 29 (64.4%) of 45 with TG alleles. SVR was induced in 20 (44.4%) with TT and in 4 (13.8%) with TG. In 18 patients with a high concentration of DNA HBV, SVR was achieved in 4 (22.2%) and in the group of 16 patients with TG — in 3 (18.7%) individuals.
The correlation between the kinetics of HBV DNA and the IFNL-3 polymorphism, was analyzed at 12 and 24 weeks of therapy with an assessment of the likelihood of achieving SVR. Of 46 patients with SNP 12979860 allele CC, a decline in HBV DNA >2 log10 at week 12 was observed in 42 (91.3%) and in 54 (88.5%)of 61 patients with CT. At 24 weeks of therapy, the decline in HBV DNA by >2 log10 in the CC group was found in 46 (100.0%) and in 61 (100.0%) patients in the CT group. SVR was achieved in 20 (43.5%) and in 11 (18.0%) persons, respectively.
At week 12 in the TT group (SNP rs8099917), a decline in HBV DNA by >2 log10 was noticed in 58 (92.0%) of 63 persons, in the group with TG alleles in 40 (88.8%) of 45. At week 24: TT — 63 (100.0%) patients and TG — 45 (100.0%) patients. SVR was achieved in 23 (36.5%) and 8 (17.7%), respectively. Predictors of HBsAg clearance were assessed depending on the baseline HBsAg concentration, qHBsAg dynamics, HBV DNA, and genetic polymorphism IFNL-3 (SNP 12979860 b SNP rs8099917).
HBsAg clearance was diagnosed in 9 (8.3%) persons, 5 females and 4 males. Portoseptal fibrosis (F3-META-VIR) was documented in only 1 patient. The rest of the patients had minimal fibrosis. The baseline low level of qHBsAg <2000 IU/ml was detected in 5 out of 9 persons (55.6%), while among 108 patients in the general group, only in 21 (19.4%). The baseline low concentration of DNA HBV <6 log10 was detected in 7 out of 9 (77.8%) persons with HBsAg clearance and in 82 (75.9%) persons in the general group.
At 12 weeks of treatment, a decline in HBsAg concentration >0.5 log10 or a negative qualitative test was documented in 8 out of 9 persons. At 24 weeks all 9 patients achieved a decline in qHBsAg >log10 concentration. At 12 and 24 weeks of treatment, a decline in HBV DNA of >2 log10 was achieved in all patients.
HBsAg clearance was observed in all patients during therapy, with seroconversion in 2 persons. In another two patients, anti-HBs seroconversion was documented 48 weeks after the end of treatment.
The presented study demonstrates the effect of genetic polymorphism IFNL-3 (SNP 12979860 and SNP rs8099917) on SVR induction and HBsAg clearance in patients with chronic HBeAg-negative hepatitis B, genotype D during therapy with peginterferon-α2a for 48 weeks. Without taking into account the genetic polymorphism of IFNL-3, one of the most significant baseline positive predictive factors of the virus was a low concentration of HBsAg (<2,000 IU/ml), as indicated by other authors [27–30]. The baseline HBsAg concentration did not depend on favorable SNP 12979860 CC and SNP rs8099917 TT or unfavorable SNP 12979860 CT and SNP rs8099917 TG genetic polymorphism. However, all patients with a baseline low HBsAg concentration and favorable IFNL-3 polymorphism achieved SVR. The baseline HBsAg concentration and the decline in this viral protein in the serum during treatment reflects the dynamics of HBV ccc DNA clearance from HBV-infected hepatocytes [31].
The baseline HBV DNA concentration had a lower predictive value in assessing the achievement of SVR than q HBsAg, as well as with taking into account the IFNL-3 polymorphism.
This work demonstrates the key effect of IFNL-3 polymorphism (SNP 12979860 and SNP rs8099917) on the kinetics of qHBsAg and HBV DNA at 12 and 24 weeks of therapy. The most significant decline in these viral markers was observed with a favorable polymorphism of SNP 12979860 alleles CC and SNP rs8099917 TT alleles. Recent studies have shown that such a response to peginterferon therapy at 12 and 24 weeks of therapy leads to an increase in the likelihood of SVR induction [30, 31]. HBsAg clearance was documented in 9 (8.3%) of 108 individuals. In 7 of them alleles of CC SNP 12979860 (77.7%) were detected in the general population of patients (n=108) in 46 (42.6%) and TT SNP rs8099917 in 8 (88.8%), in the general group — in 63 (58.3%).
A meta-analysis conducted in recent years on the effect of IFNL-3 polymorphism (SNP 12979860 and SNP rs8099917) on SVR achievement and HBsAg clearance during peginterferon therapy confirmed this close relationship [9, 32, 33]. And although the identification of IFNL-3 polymorphism is not recommended by any of the national protocols for the treatment of CHB, the favorable genetic polymorphism SNP 12979860, the CC allele is widely used in clinical practice as a positive predictive factor, as rescue therapy with Peg-INF for selected patients with resistance (YMDD-mutations) to nucleoside analogs [34].
The authors declare no conflict of interest.
References
- Ekstrom , Kumar R., Zhao Y., Ling Yee M. et al. Real world experience with pegylated interferon and ribavirin in hepatitis C genotype 1 population with favourable IL28B polymorphism . Gastroenterol Rep (Oxf). 2017; 5(3):208–212. doi: 10.1093/gastro/gow033.
- Fedorchenko S. V., Klymenko B., Martynovich T. L., Lyashok O. V., Yanchenko V. I. IL-28B genetic variation, gender, age, jaundice, hepatitis C virus genotype, and hepatitis B virus and HIV co-infection in spontaneous clearance of hepatitis C virus. Turkish Journal of Gastroenterology. 2019. 30(5). 436–444. doi: 10.5152/tjg.2019.18328.
- Fedorchenko S. V. Coinfekciya HCV/HBV. [Coinfection HCV/HBV.]. Kiev Monograph VSI “Medicina”, 2018, 120
- Zhao , Zhang X., Fang L. et al. Association between IL28B Polymorphisms and Outcomes of Hepatitis B Virus Infection: A meta-analysis .BMC Med Genet. 2020; 21(1):88. doi: 10.1186/s12881-020-01026-w.
- Regina Souza da Silva Conde S., Luciana L. Rocha, Ferreira V. M. et Absence of correlation between IL-28B gene polymorphisms and the clinical presentation of chronic hepatitis B in an Amazon Brazilian population .Dis Markers. 2014; 534. doi: 10.1155/2014/534534.
- Mehdizadeh Baghbani. J., Mirnajd Gerami. S., Ghojazadeh M. et al. Interleukin 28B Genetic Polymorphism and Spontaneous Recovery from Hepatitis B Virus Infection in an Iranian Azeri Population . Hepatitis Monthly: 2017; 17 (9); e11706 doi: 5812/hepatmon.11706.
- Holmes A., Nguyen T., Ratnam D. et al. // IL28B genotype is not useful for predicting treatment outcome in Asian chronic hepatitis B patients treated with pegylated interferon-α. J Gastroenterol Hepatol. 2013 May; 28(5):861-6. doi: 10.1111/jgh.12110.
- Wei L., Wedemeyer H., Liaw Y. F. et No association between IFNL3 (IL28B) genotype and response to peginterferon alfa-2a in HBeAg-positive or -negative chronic hepatitis B. PLoS One. 2018 Jul; 17; 13(7): e0199198. doi: 10.1371/journal.pone.0199198.
- Zhifeng Lin, Junguo Zhang, Xiaomin Ma et al. The Role of Interferon Lambda 3 Genetic Polymorphisms in Response to Interferon Therapy in Chronic Hepatitis B Patients: An Updated Meta-Analysis . Hepat 2016 Jul; 16(7): e37534. doi: 10.5812/hepatmon.37534.
- MacLachlan J. H., Cowie B. C. Hepatitis B Virus Epidemiology. Cold Spring Harbor Perspectives in Medicine. May 2015; 5(5):a021410-a02141 doi:10.1101/cshperspect.a021410.
- Alexopoulou A., Karayiannis P. HBeAg negative variants and their role in the natural history of chronic hepatitis B virus infection . World J 2014 Jun 28; 20(24):7644-52. doi: 10.3748/wjg.v20.i24.7644.
- Griffiths J., Dunnigan C. M., Russell C. D., Haas J.G.J. The Role of Interferon-λ Locus Polymorphisms in Hepatitis C and Other Infectious Diseases. Innate Immun. 2015;7: 231-242. doi.org/10.1159/000369902 .
- Willem Brouwer. P. A., Rijckborst V. et al. Polymorphisms near the IL28B Gene Are Not Associated with Response to Peginterferon in HBeAg-Negative Chronic Hepatitis B Patients. Journal of Hepatology. April 2013; 58: S299-S299. doi:10.1016/S0168-8278(13)60739-4.
- Mangia A., Santoro R., Mottola L. et al Lack of association between interleukin 28B variant and HBsAg clearance after interferon Journal of Hepatology. 2011; 54: S 525 (Poster).
- Sonneveld J., Vincent W.,Wong S., Woltman A. M. et al. Polymorphisms Near IL28B and Serologic Response to Peginterferon in HBeAg-Positive Patients With Chronic Hepatitis B. Gastroenterology. Volume 142, Issue 3, March 2012, Pages 513-520. doi.org/10.1053/j.gastro.2011.11.025.
- Lampertico P., Viganò M., Cheroni C. et al. // IL28B polymorphisms predict interferon-related hepatitis B surface antigen seroclearance in genotype D hepatitis B e antigen-negative patients with chronic hepatitis Hepatology. 2013 Mar; 57(3):890-6. doi: 10.1002/hep.25749.
- Boglione L., Cusato J., Allegra S. et al. // Role of IL28-B polymorphisms in the treatment of chronic hepatitis B HBeAg-negative patients with peginterferon. Antiviral Res. 2014 Feb;102:35–43. doi: 1016/j. antiviral.2013.11.014.
- Fedorchenko S. V. Hronicheskay HDV-infekcia. [Chronic HDV-infection.]. Kiev Monograph VSI “Medicina”, 2014, 152.
- EASL 2017 Clinical Practice Guidelines on the managemen to hepatitis B virus Journal of Hepatology. 2017.vol. 67. 370–398.
- Antiviral treatment for chronic hepatitis B. NICE Pathway last updated: 10 August P. 1–13.
- Goulis , Karatapanis S., Akriviadis E. A. et al. On treatment prediction of post treatment sustained response to peginterferon alfa-2a for HBeAg-negative Patients Chronic Hepatitis B (CHB) Patients using HBsAg and HBV DNA levels at weeks 12 and 24: PERSEAS cohort final results. Hepatology. 2013. Vol.58: 697A-698A, pp. 697A–698A.
- Moucari , Mackiewicz V., Olivier L. et al. Early serum HBsAg drop: a strong predictor of sustained virological response to pegylated interferon alfa-2a in HBeAg-negative patients. Hepatology. 2009 Apr; 49(4):1151-7. doi: 10.1002/hep.22744.
- Marcellin , Bonino F., Yurdaydin C. et al. Hepatitis B surface antigen levels: association with 5-year response to peginterferon alfa-2a in hepatitis B e-antigen-negative patients. Hepatol Int. 2013 Mar;7(1):88-97. doi: 10.1007/s12072-012-9343-x.
- Wu , Luo W., Wu Y. et al. HBsAg quantification predicts off-treatment response to interferon in chronic hepatitis B patients: a retrospective study of 250 cases. BMC Gastroenterology. 2020 Apr 21; 20(1):121. doi: 10.1186/s12876-020-01263-6.
- Takkenberg B., Jansen L., De Niet A. et al. Baseline hepatitis B surface antigen (HBsAg) as predictor of sustained HBsAg loss in chronic hepatitis B patients treated with pegylated interferon-α2a and adefovir. Antivir Ther. 2013; 18(7):895-904. doi: 10.3851/IMP2580.
- Lampertico P., Messinger D., Cornberg M. et al. A genotype-specific baseline score predicts post-treatment response to peginterferon alfa-2a in hepatitis B e antigen-negative chronic hepatitis B. Ann 2018; 31 (6):712-721. doi: 10.20524/aog.2018.0300.
- Vlachogiannakos J., Papatheodoridis G. V. et al. HBeAg-negative chronic hepatitis B: why do I treat my patients with pegylated interferon-alfa? Liver 2014, 34:127–132. doi: 10.1111/liv.12404.
- Lampertico P., Messinger D., Oladipupo et al. An easy-to-use baseline scoring system to predict response to peginterferon alfa-2a in patients with chronic hepatitis B in resource-limited settings. Antivir Ther. 2018; 23(8):655-663. doi: 10.3851/IMP3251.
- Ming-Hui Li, Lu Zhang , Xiao-Jing Qu et Kinetics of Hepatitis B Surface Antigen Level in Chronic Hepatitis B Patients who Achieved Hepatitis B Surface Antigen Loss during Pegylated Interferon Alpha-2a Treatment Chin Med J (Engl). 2017 Mar 5; 130 (5):559–565. doi: 10.4103/0366-6999.200554.
- Chuaypen , Posuwan N., Chittmittraprap S. et al. Predictive role of serum HBsAg and HBcrAg kinetics in patients with HBeAg-negative chronic hepatitis B receiving pegylated interferon-based therapy. Clin Microbiol Infect. 2018 Mar; 24(3):306.e7-306.e13. doi: 10.1016/j.cmi.2017.07.016.
- Chuaypen , Sriprapun M., Praianantathavorn K. et al. Kinetics of serum HBsAg and intrahepatic cccDNA during pegylated interferon therapy in patients with HBeAg-positive and HBeAg-negative chronic hepatitis B. J Med Virol. 2017 Jan; 89(1):130-138. doi: 10.1002/jmv.24601.
- Chong Zhang, Zhengrong Yang, Ziyi Wang et HBV DNA and HBsAg: Early prediction of response to peginterferon α-2a in HBeAg-negative chronic hepatitis B. Int J Med Sci. 2020; 17(3): 383–389. doi: 10.7150/ ijms.39775.
- Sang Yu , Yao-Ren H., Guo-Sheng G. et al. Interleukin-28B Polymorphisms predict the efficacy of peginterferon alpha in patients with chronic hepatitis B: a meta-analysis. Frontiers in Medicine. 2021 July; 8. article 691365.
- Stanzione M., Stornaiuolo G., Rizzo V. et al. HBsAg seroconversion after pegylated interferon alfa 2a rescue in a lamivudine-resistant patient with HBeAg-negative chronic hepatitis B and favourable IL28-B genotype. Infez 2016 Jun 1; 24(2):144-6. PMID: 27367326.
Information about the authors:
Fedorchenko S. V. — doctor of medical sciences, head of the department Viral Hepatitis and AIDS, SI “L. V. Hromashevskyi institute of epidemiology and infection diseases of NAMS of Ukraine”. ORCID: 0000 0002 5338 7072
Martynovych T. L. — PhD of medicine, Department of Viral Hepatitis and AIDS, SI “L. V. Hromashevskyi institute of epidemiology and infection diseases of NAMS of Ukraine”. ORCID: 0000 0002 3046 7993
Klimenko Zh. B. — PhD of medicine, Department of Viral Hepatitis and AIDS, SI “L. V. Hromashevskyi institute of epidemiology and infection diseases of NAMS of Ukraine”. ORCID: 0000 0002 3160 7740
Liashok O. V. — PhD of medicine, Department of Viral Hepatitis and AIDS, SI “L. V. Hromashevskyi institute of epidemiology and infection diseases of NAMS of Ukraine”. ORCID: 0000 0002 2857 292X
Solianyk I. V. — Department of Viral Hepatitis and AIDS, SI “L. V. Hromashevskyi institute of epidemiology and infection diseases of NAMS of Ukraine”.
Reznyk V. A. — Department of Viral Hepatitis and AIDS, SI “L. V. Hromashevskyi institute of epidemiology and infection diseases of NAMS of Ukraine.
Відомості про авторів:
Федорченко С. В. — д. м. н., завідувач відділу вірусних гепатитів та СНІДу ДУ «Інститут епідеміології та інфекційних хвороб ім. Л. В. Громашевського НАМН України».
ORCID: 0000 0002 5338 7072
Мартинович Т. Л. — к. м. н., старший науковий співробітник відділу вірусних гепатитів та СНІДу ДУ «Інститут епідеміології та інфекційних хвороб ім. Л. В. Громашевського НАМН України». ORCID: 0000 0002 3046 7993
Клименко Ж. Б. — к. м. н., старший науковий співробітник відділу вірусних гепатитів та СНІДу ДУ «Інститут епідеміології та інфекційних хвороб ім. Л. В. Громашевського НАМН України». ORCID: 0000 0002 3160 7740
Ляшок О. В. — к. м. н., старший науковий співробітник відділу вірусних гепатитів та СНІДу ДУ «Інститут епідеміології та інфекційних хвороб ім. Л. В. Громашевського НАМН України». ORCID: 0000 0002 2857 292Х
Соляник І. В. — лікар-інфекціоніст відділення вірусних гепатитів та СНІДу ДУ «Інститут епідеміології та інфекційних хвороб ім. Л. В. Громашевського НАМН України».
Резник В. А. — лікар-інфекціоніст відділення вірусних гепатитів та СНІДу ДУ «Інститут епідеміології та інфекційних хвороб ім. Л. В. Громашевського НАМН України.
