Реверсія чутливості штамів ентерококів до антибіотиків при їх культивуванні в культурах клітин людини і тварин
УДК 616.002.828:[577.181.5+62-581/584]+57.017.4
Д. П. Єгоров¹, C. Л. Рибалко², С. М. Григор’єва², Д. Б. Старосила², В. П. Широбоков¹
1. Національний медичний університет імені О. О. Богомольця, Київ, Україна
2. ДУ «Інститут епідеміології та інфекційних хвороб імені Л. В. Громашевського НАМН України», Київ, Україна
Реверсія чутливості штамів ентерококів до антибіотиків при їх культивуванні в культурах клітин людини і тварин
Одним із критеріїв оцінки пробіотиків є властивість їх антибіотикорезистентності, що повинна бути характеристикою відбору перспективних для виробничої технології штамів. Але ці властивості здатні до значного варіювання, наприклад, може відбуватися їх втрата при технологічних пасажах, або може бути присутня так звана набута резистентність (плазмідна). Плазмідна резистентність зумовлена присутністю R-плазмід та може виникати при антибіотикотерапії, хіміотерапії, променевій терапії макроорганізму.
У роботі представлено результати визначення чутливості штаму ентерококу, виділеного з препарату-пробіотика «Лінекс», та ентерококів, ізольованих від новонароджених дітей, при їх культивуванні у культурах клітин, які слугували моделлю макроорганізму. Встановлено, що після культивування пробіотичного штаму ентерококів Enterococcus faecium та клінічного штаму Enterococcus faecalis у культурах клітин людини та тварин відбуваються зміни у розмірах зон затримки росту штамів навколо дисків з антибіотиками, що може вказувати на реверсію їх чутливості та резистентності до антибіотиків.
Ключові слова: ентерококи, антибіотики, реверсія, культури клітин.
D. P. Yehorov¹ , C. L. Rybalko², S. M. Grigorieva² , B. Starosila², V. P. Shirobokov¹
Reversal of enterococci strains antibiotic sensitivity during their cultivation in human and animal cell cultures
1. О. О. Bogomolets National Medical University, Kyiv, Ukraine
2. SІ “L. V. Gromashevsky Institute of Epidemiology and Infectious Diseases of the National Academy of Medical Science of Ukraine”, Kyiv, Ukraine
One of the crucial criteria for probiotics evaluation is their property of antibiotic resistance, which should be characteristic for the selection of the promising strain for production technology. But these properties are capable of significantly varying, for example, their loss may occur during technological passages, and
acquired resistance (plasmid) may be present. Plasmid resistance appears due to the presence of R-plasmids and can occur during antibiotic therapy, chemotherapy, and radiation therapy.
This paper presents the results of determining the sensitivity of the enterococcus strain isolated from the probiotic “Linex” and enterococci isolated from newborn children before and after cultivation in cell cultures, which were used as a model. It was established that after the cultivation of a probiotic strain of Enterococcus faecium and the clinical strain of Enterococcus faecalis in human and animal cell cultures, there are changes in the strain’s diameters of the growth inhibition zones around the disks with antibiotics, which may indicate a reversal of their sensitivity and resistance to antibiotics.
Key words: enterococci, antibiotics, reversion, cell cultures.
To determine the natural sensitivity of enterococcal strains isolated from the probiotic preparation and enterococci isolated from newborn children, the method of determining the sensitivity of bacteria and fungi to antibiotics when cultivated in human and animal cells was used (1). Usually, diagnostic laboratories use the standard disk-diffusion method for determining the sensitivity of microorganisms to antibiotics. But it is known that when microbial cells interact with cells of a macroorganism, the adhesive properties of bacteria can change, which could lead to a change in the antimicrobial sensitivity of bacteria. The phenomenon of reversal of sensitivity to antibiotics was first discovered in lactic acid bacteria during their interaction with human lymphoblastoid cells.
The purpose of the research was the determination of the natural sensitivity of clinical strains of enterococci isolated from newborn children and the strain of enterococci isolated from the probiotic drug “Linex” during cultivation in cell cultures.
The objects of research were strains of enterococci of the Enterococcus faecalis species, isolated from the biotopes of newborn children (navel, stomach contents, intestinal contents) and the strain of enterococci — Enterococcus faecium, isolated from the probiotic drug “Linex”. Enterococcal agar and Muller-Hinton agar were used to preserve the biological activity of the studied strains. The presence of changes in the sensitivity of the strains was studied after their cultivation in cell cultures: HEp-2 — human tumour cell line; BHK — Syrian hamster kidney cells; MDCK — dog kidney cells; RK-13 — rabbit kidney cells. A suspension of cells of the studied bacteria (at a concentration of 1.0×108 CFU/ml — 0.5 units according to the McFarland standard) was inoculated into tissue cultures. Susceptibility to antibiotics was studied before cultivation in cell cultures (initial) and after (final) using commercially produced discs ( HiMedia, India; “Aspect”, Ukraine).
Research materials and methods. Microorganism strains: Enterococcus faecium, isolated from “Linex”; clinical strain Enterococcus faecalis isolated from newborn babies. Cell cultures: HEp-2; BHK; MDCK; RK-13. RPMI-1640 medium for growing cell cultures without the addition of antibiotics. Nutrient media for enterococci and determining antibiotic sensitivity: enterococcal agar, Muller-Hinton agar. Disks with antibiotics manufactured by Himedia, India and “Aspect”, Ukraine, registered in Ukraine: aminoglycosides (gentamicin, amikacin); cephalosporins (ceftazidime, ceftriaxone, cefuroxime, cefepime); vancomycin, linezolid, amoxicillin; oxacillin, benzylpenicillin; macrolides — azithromycin; tigecycline, lincomycin, clindamycin, furazidin; fluoroquinolones (ciprofloxacin, levofloxacin). The disc-diffusion method was used for the study. Control was carried out with standard test cultures: Escherichia coli 25922, S. aureus ATCC 25923 and P. aeruginosa ATCC 27853. Depending on the diameter of the growth inhibition zone of the tested bacteria around the discs with antibiotics, the studied strains were divided into three groups: sensitive — S; resistant — R, and intermediate — I.
Results and discussion. The investigated strain of Enterococcus faecium was pre-cultivated on enterococcal agar, the isolated colony was selected and screened on simple nutrient agar in a test tube. The culture grown after 24 hours of incubation in a thermostat at a temperature of +37 °C was used to study its sensitivity to antibiotics. A suspension of cells of the studied bacteria (at a concentration of 0.5 Mcfarland units) was inoculated on the Muller-Hinton medium, and discs with antibiotics were applied. Growth inhibition zones were measured after 18–24 hours. The obtained data are listed in Table 1.
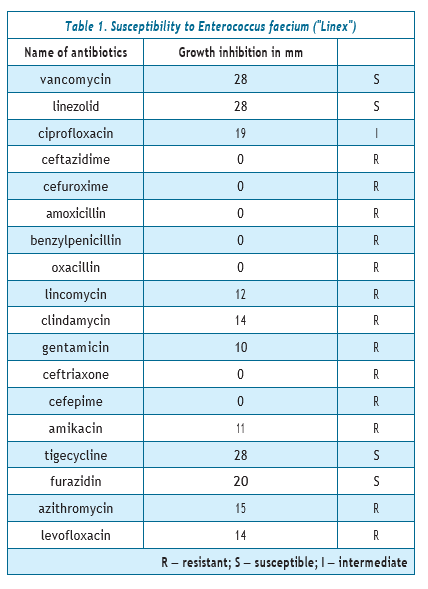
The investigated strain of Enterococcus faecium was resistant to 13 antibiotics: 3rd generation cephalosporins: ceftazidime, cefuroxime, ceftriaxone; 4th generation — cefepime; also to amoxicillin, benzylpenicillin, oxacillin, lincomycin, clindamycin, 2nd and 3rd generation aminoglycosides: gentamicin and amikacin; to macrolide — azithromycin. Sensitivity was found to vancomycin, linezolid, tigecycline and furazidin. Enterococcus faecium was moderately resistant to ciprofloxacin and resistant to levofloxacin.
Enterococcus faecium, isolated from enterococcal agar and previously grown on nutrient agar in a test tube, was used for introduction into cell cultures. Next, the Enterococcus faecium strain was inoculated in cultured monolayer cell lines for 24 hours. For this purpose, a 1 cm3 suspension of microorganisms (at a concentration of 1.0×108 CFU/ml — 0.5 units according to the McFarland standard) was inoculated into cell cultures and cultivated in RPMI-1640 medium without the addition of serum and antibiotics in a thermostat at a temperature of ±37 °C.
As a control, a suspension of Enterococcus faecium was used (at a concentration of 0.5 units according to the McFarland standard).
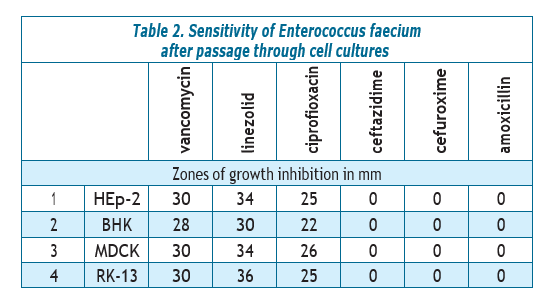
E.faecium was re-isolated from the cell cultures after 24 hours of incubation and it was studied whether the indicators of sensitivity to antibacterial drugs had changed
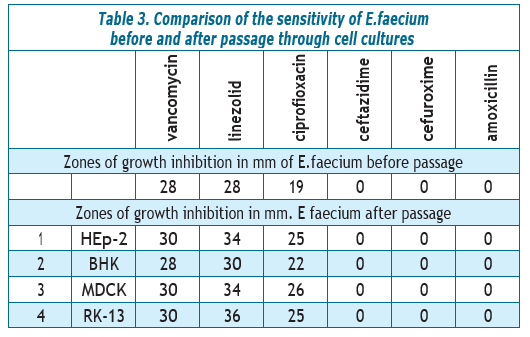
As can be seen from the table, the growth inhibition zone of E.faecium after passage through cell culture HEp-2 increased by 2 mm to vancomycin, by 4–6 mm to linezolid, by 5–6 mm to ciprofloxacin (from I to S); E.faecium remained stably resistant to ceftazidime, cefuroxime and amoxicillin. After passage through the culture of BHK cells, the parameters did not change, with the exception of sensitivity to ciprofloxacin, which increased by 2 mm, thus from moderately sensitive E.faecium became sensitive (from I to S).
In MDCK and RK-13 cell cultures, an increase in the diameters of the growth inhibition zone of E.faecium was also noted by 2 mm to vancomycin, by 4–6–8 mm to linezolid, by 6–7 mm to ciprofloxacin (from I to S); to ceftazidime, cefuroxime and amoxicillin, E.faecium remained stably resistant as well.
The next stage of research was to determine the sensitivity of the clinical strain of E. faecalis before and after passage through cell cultures.
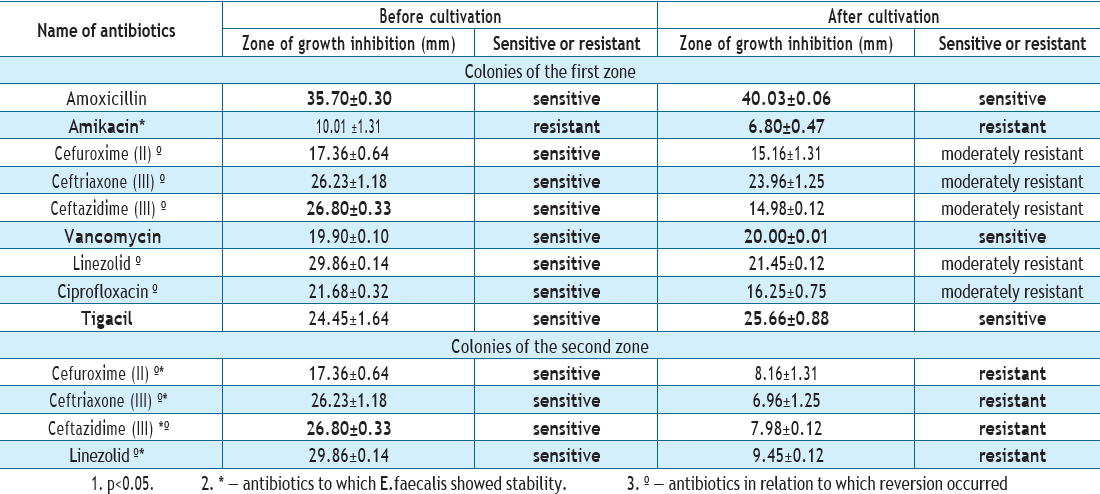
Analysis of the data provided in Table 4 showed that in the case of passaging through RK-13 cell culture, two zones of growth inhibition of the E.faecalis strain were formed. Colonies enterococci of the first zone have changed their sensitivity to 2nd and 3rd generation cephalosporins: ceftazidime, cefuroxime, and ceftriaxone for moderate resistance. E.faecalis zones of growth inhibition decreased by 8–9 mm to linezolid (that is, it turned from sensitive to moderately resistant); by 5–6 mm to ciprofloxacin (from S to I as well). In relation to the strain remained sensitive to vancomycin, amoxicillin, and tigecycline; to amikacin, it is still stably resistant.
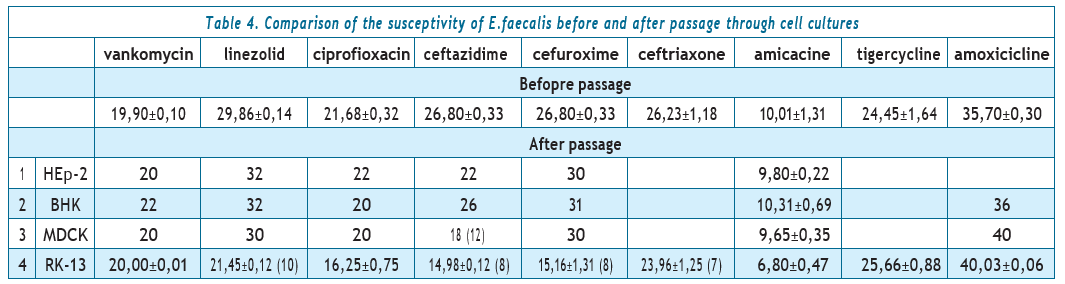
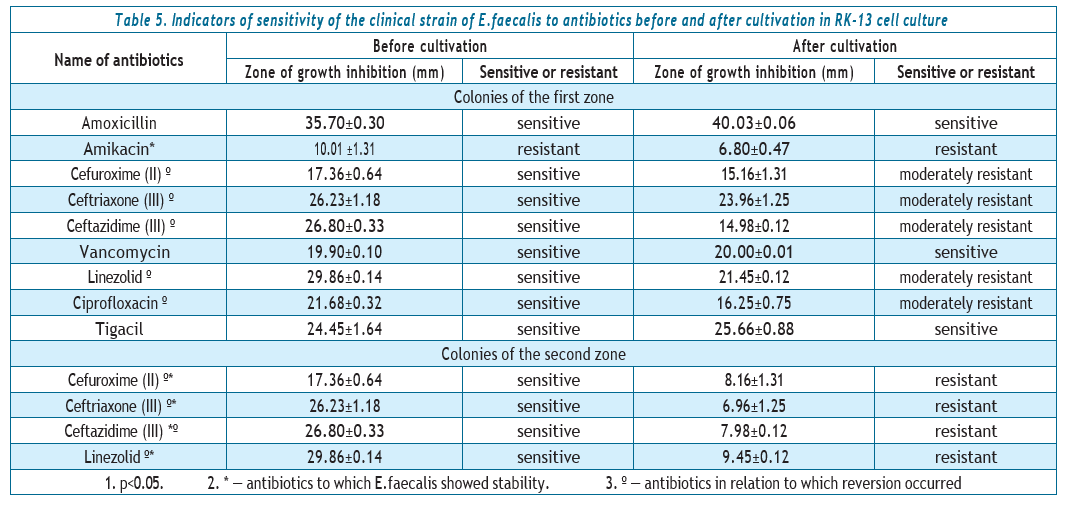
Colonies of the second zone due to reversion changed their sensitivity to resistance to drugs ceftazidime, cefuroxime, ceftriaxone and linezolid..
Colonies of the first zone of enterococci changed their sensitivity to 2nd and 3rd generation cephalosporins: ceftazidime, cefuroxime, ceftriaxone to moderate resistance. Zones of growth inhibition of E. faecalis decreased by 8–9 mm to linezolid (that is, they turned from sensitive to moderately resistant); by 5–6 mm to ciprofloxacin (from S to I as well). The strain remained sensitive to vancomycin, amoxicillin, and tigecycline; stably resistant to amikacin.
Colonies of the second zone due to reversion changed their sensitivity to resistance to the drugs ceftazidime, cefuroxime, ceftriaxone and linezolid.
Conclusions. After the cultivation of enterococcal strains in transplanted cultures of animal and human cells, their sensitivity to antibiotics was changed. This property has been found to be unstable: after passages, the sensitivity and resistance of the studied microorganisms are reversed. The same processes can occur in the human body and lead to ineffective treatment with antibiotics, the sensitivity to which is determined by traditional methods.
Literature
- Yegorov P., Rybalko S. L., Grigorʼeva S. M., Starosila D. B., Shirobokov P. Changes in sensitivity to antibiotics in bacterial strains during their co-cultivation with human and animal cell cultures // Превентивна медицина. Теорія і практика., 2023. – 2(2), С. 24–27.
- Rybalko S. L., Pokas Ye. V., Dieyev V. A., Liaskovski Т. M., Furzikova Т. , Diadiun S. T., Ivanskaya N. V., Nastoyashcha N. I., Verkhatsky P. P., Salkov S. T. Antibiotic resistance changes in strains of bacteria and yeast-like fungi following their growth in established cell lines of human and animal origin // ISSN 0233-7657. Biopolymers and the cell. 2006. V. 22. № 5.
- Rybalko S. L., Liaskovski Т. M., Podgorskyi V. S., Harmasheva I. L., Kovalenko N. K. Reversal of antibiotic sensitivity of lactic acid bacteriae in transplanted cultures of human lymphoblastoid cells. Microbiological 2006. V.68, №6. P. 43–51;
- Rybalko L., Pokas Ye. V., Dieyev V. A., Liaskovski Т. M., Diadiun S. T., Saenko V. F. The method of changing antibiotic resistance of enterobacteria to antibiotic sensitivity. Ukrainian patent for a utility model № 20587 dated 15.01.2007year.
- Anvar Y., van Biesen М., Dasgupta К. Ineraction of biofilm bacteria with antibiotics in a novel in vitro chemostat system. Antimicrob. Agents 1989. 33, P. 1824–1826.
- Anvar , Costerton J. W. Effective use of antibiotics in the treatment of biofilm-associated infections. ASM News, 1992. 58, P. 665–668.
- Gilbert P., Collier J., Brown M. R. Influence of growth rate on susceptibility to antimicrobial agents: biofilms, cell cycle, dormancy, and stringent Antimicrob. Agents Chemother. 1990. 34, P. 1865–1868.
- Costerton , Cheng K.-J. Geesey G. G., Anvar H. Bacterial biofilms in nature and disease. Ann. Rev. Microbiol. 1987, 41. P. 435–464.
- Gilbert P. Attachment and biofilm formation: the critical event in microbial J. Pharm. Pharmacol. 1997, 49. suppl. 4. P. 8.
- Golubev D. B., Sominina A. A., Medvedeva M. N. Guidelines for the use of the cell cultures in Leningrad, Medicine, 1976. P. 54.
- National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial susceptibility testing; eleventh informational New York, 2001. V. 2.79 y.
- WHO Technical Report Series (criteria for interpreting test results based on the Bauer-Kirbi method). Geneva, N 673. P. 147–169.
- Onveji С. О., Nicolau D. P., Nightingale С., Bow L. Interferon-gamma effects on activities of gentamicin and vancomycin against Enterococcus faecalis resistant to the drugs: an in vitro study with human Int. J. Antimicrob. Agents. 1999. П. P. 31–37.
- Ouadrhiri Y., Scorneaux В., Sibille У., Tulkens P. M. Mechanism of the intracellular killing and modulation of antibiotic susceptibility of Listeria monocytogenes in THP-1 macrophages activated by gammainterferon. Antimicrob. Agents Chemother. 1999. 43. P. 1242–1251.
- Difco Manual Dehydrated culture media and reagents for microbiology: Tenth Detroit, 1994. P. 844—850. УДК 616.002.828: [577.181.5+62-581 /584] +57.017.4.
- Zhalko-Titarenko V. P., Bondarenko V. N., Grigoriev A. V. Dynamics of Shigella interaction with Epithelium during infection. Journal of Microbiology, Epidemiology and 1986. № 4, P. 21-24.
Відомості про авторів:
Єгоров Д. П. – Національний медичний університет імені О. О. Богомольця, кафедра мікробіології,вірусології та імунології, асистент кафедри.
E-mail: grigav@gmail.com.
Широбоков В. П. – д. м. н., професор, академік НАН та НАМН України, Національний медичний університет імені О. О. Богомольця, кафедра мікробіології, вірусології та імунології, завідувач кафедри.
E-mail: v.p.shyrobokov@gmail.com, ORCID: 0000-0003-0882-148X.
Рибалко С. Л. – д. м. н., професор, ДУ «Інститут епідеміології та інфекційних хвороб ім. Л. В. Громашевського НАМН України», лабораторія експериментальної хіміотерапії вірусних інфекцій, завідувач лабораторії. E-mail: y_dasha@ukr.net,
ORCID: 0000-0002-1913-1380
Григорʼєва С. М. – к. м. н., ДУ «Інститут епідеміології та інфекційних хвороб ім. Л. В. Громашевського НАМН України», лабораторія експериментальної хіміотерапії вірусних інфекцій, с.н.с.
E-mail: Grigorevasm@ukr.net.
Старосила Д. Б. – к. б. н., ДУ «Інститут епідеміології та інфекційних хвороб ім. Л. В. Громашевського НАМН України», лабораторія експериментальної хіміотерапії вірусних інфекцій, с.н.с.
E-mail: y_dasha@ukr.net.
Information about the authors:
Yehorov D. P. – Bogomolets national medical university, department of microbiology, virology and immunology, assistant of the department, Kyiv, Ukraine.
E-mail: grigav@gmail.com.
Shyrobokov V. P. – Doctor of medical science, professor, academician of the National Academy of Sciences and National Academy of Sciences of Ukraine, Bogomolets national medical university, department of microbiology, virology and immunology, head of the department.
E-mail: v.p.shyrobokov@gmail.com, ORCID: 0000-0003-0882-148X.
Rybalko S. L. – Doctor of medical science, professor, head of the laboratory of experimental chemotherapy of viral infection of the SІ “L. V. Gromashevsky Institute of Epidemiology and Infectious Diseases of the National Academy of Sciences of Ukraine”, Kyiv, Ukraine.
E-mail: y_dasha@ukr.net, ORCID: 0000-0002-1913-1380.
Hryhorieva S. M. – Candidate of medical science, senior researcher of the laboratory of experimental chemotherapy of viral infection of the SІ “L. V. Gromashevsky Institute of Epidemiology and Infectious Diseases of the National Academy of Sciences of Ukraine”, Kyiv, Ukraine.
E-mail: Grigorevasm@ukr.net.
Starosyla D. B. – Candidate of biology science, senior researcher laboratory of experimental chemotherapy of viral infection of the SІ “L. V. Gromashevsky Institute of Epidemiology and Infectious Diseases of the National Academy of Sciences of Ukraine”, Kyiv, Ukraine.
E-mail: y_dasha@ukr.net.
